To have real impact, research needs to be undertaken with purpose, integrity and effective planning and management.
The Research and Innovation Office manages research at Murdoch University, from grants and contracts to systems, ethics and commercialisation. The office works with researchers through the whole journey, ensuring people work in a safe environment, underpinned by an ethical framework.
The Research and Innovation Office manages:
We support Murdoch researchers in the development, preparation and submission of research grant applications, oversee negotiation and ongoing administration of research awards, collaborative agreements and contracts.
Our expertise in research funding opportunities across Australian Competitive Grants, charities, government, learned societies and other bodies enables us to support researchers to apply for support and resources.
We also share our expertise in the form of information, seminars and workshops, to help improve the quality of research applications from Murdoch. As well as this, we manage internal research support schemes including Small Grants, the Sir Walter Murdoch Distinguished Adjunct Professorship and Distinguished Collaborator and Strategic Research Fund.
If you would like to know more about these services, please contact our Research Grants and Contracts team on researchsupport@murdoch.edu.au.
Building on the traditional academic transfer of knowledge via publication, education and training, we have a specific focus on industry collaborative research, connecting researchers and industry partners.
We raise awareness of knowledge transfer options and identify and protect commercially valuable Murdoch University Intellectual Property, supporting commercialisation, the licensing of university intellectual property and development of spin-off companies.
Helping to promote Murdoch's innovations, we assist with collaborative Research and Development for licensing, and help prepare business plans, marketing evaluations and external funding options.
Murdoch University is committed to the highest standards of responsible research conduct.
We comply with the Australian Code for the Responsible Conduct of Research and promote the Code's principles through our policies and practice.
This ensures everything we do is done safely, ethically, with academic integrity, transparency, reporting accountability and fiscal responsibility.
The Graduate Research Office is responsible for the admission and management of research students, including enrolment and re-enrolment, candidature management, scholarships and thesis examination administration.
Welcome to the Murdoch University Institutional Biosafety Office. The Office is committed to promoting a safe research environment by fostering a culture of responsible practise that is compliant with institutional biosafety requirements and regulations.
About Us The Institutional Biosafety Office (IBO) oversees the safe use of biological materials in research and teaching. Our team provides guidance on risk management, regulatory compliance, and best practices for handling biohazardous materials. We work closely with researchers, students, and staff to ensure all activities meet local and national safety standards.
Biosafety Committee The Institutional Biosafety Committee (IBC) reviews and approves research involving biohazards, including genetically modified organisms (GMOs), infectious agents, and biological toxins. The committee consists of scientists, biosafety experts, and community representatives to ensure diverse, unbiased perspectives and thorough risk assessment to protect our community from potential risks and exposure.
Services and Support
- Risk Assessments: Assistance with identifying and mitigating biological risks.
- Training & Workshops: Biosafety training for researchers, staff, and students.
- Review: Support with preparing and submitting biosafety protocols and procedures.
- Incident Management: Guidance on reporting and response to biosafety incidents.
- Liaison: The IBO acts as the liaison between Murdoch researchers, the OGTR and other relevant statutory bodies.
Resources Access our library of guidelines, templates, and forms, including:
- Biosafety manuals and standard operating procedures (SOPs)
- Risk assessment templates
- Permit application forms
- Training materials
Compliance & Regulations The IBO is required to ensure compliance with biosafety legislation, regulations and standards, including:
- The Australian Gene Technology Act 2000
- OGTR
- WA Health and Safety Act
- Defence and Strategic Goods List (DSGL)
- Security Sensitive Biological Agents (SSBA)
- University-specific biosafety policies
Application for a Generic Import Permit
Provided here is a copy of Murdoch’s Generic Import Permit listing the categories of biological materials it covers. If you wish to use this permit then please complete this MS Forms application (MU Generic Import Permit)

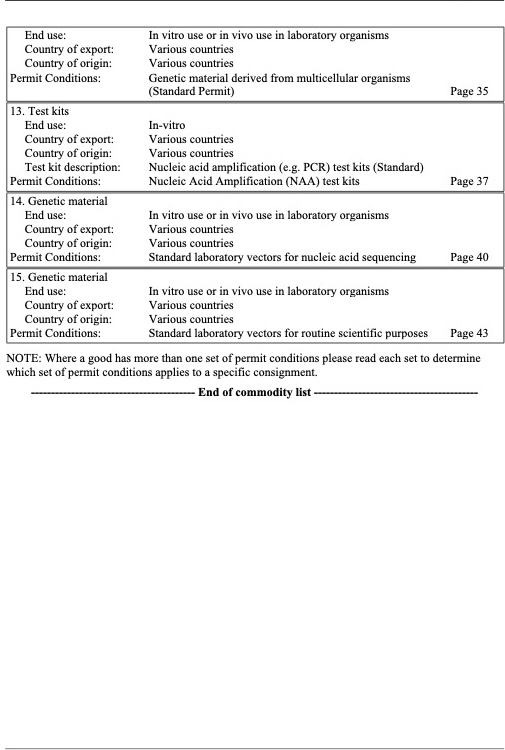
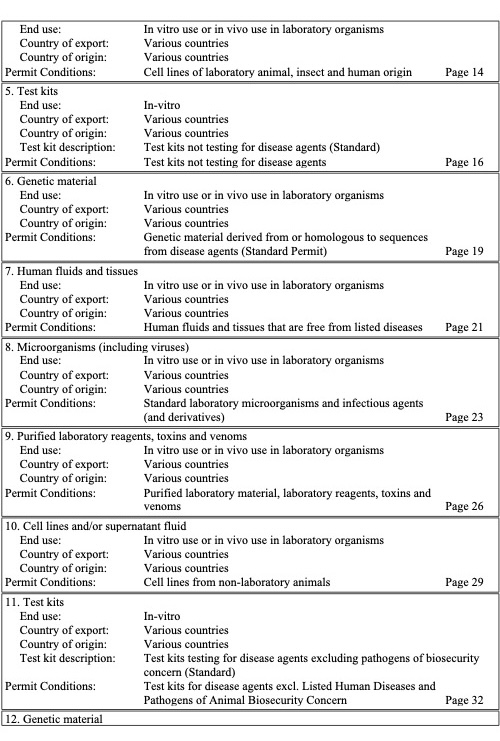
If the goods you wish to import are not listed in MU’s generic permit then you will need to apply for one specific to your project via the Biosecurity Import Conditions System (BICON).
This information is necessary to meet DAFF compliance and audit requirements. Once the request form is complete, the IBC will review the provided information and then provide a complete copy of the permit once all is in order.
Latest research news
See how our research is leaving an indelible mark on the world around us
