News
COVID-19 researchers discover hidden natural immune defence pathway

An international cohort of scientists researching COVID-19 has uncovered a hidden part of the human immune system that creates anti-viral agents.
The discovery, published in the Journal of Proteome Research, will enable the development of new tests for general active viral infection and new ways to create anti-viral drugs that extend beyond COVID-19.
Led by Professor Julien Wist and Professor Jeremy Nicholson at the Australian National Phenome Centre (ANPC) at Murdoch University in Perth, Western Australia, researchers from New Zealand, United States, United Kingdom and Germany were analysing blood and urine samples from COVID-19 patients when they uncovered a previously largely unknown piece of a natural immunity system that generates drug-like metabolites that act as anti-viral agents.
Professor Nicholson, the ANPC’s Director, said they detected 10 new compounds made by the body in response to the SARS Cov-2 virus.
The discovery of the Extended VIPERIN Pathway (X-VIP) has greatly extended the knowledge of the VIPERIN anti-viral pathway, part of the innate immune system that reacts to most viral infections.
VIPERIN – short for virus inhibitory protein, endoplasmic reticulum-associated, interferon-inducible - is a multifunctional, interferon-inducible protein that regulates virus replication. When a virus attacks the body, it triggers an interferon response, and VIPERIN is believed to suppress virus replication.
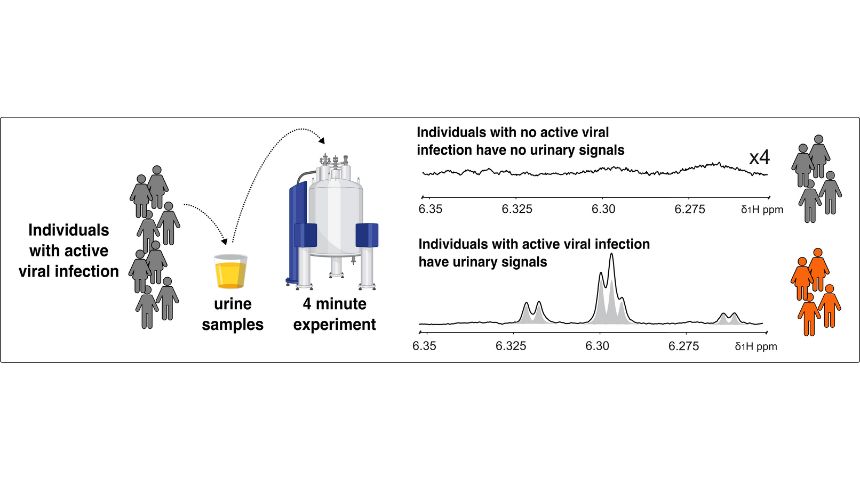
Image: Individual with a suspected infection be screened within minutes by simply providing a urine sample. The urine of individuals with active infection will display a characteristic serie of signals as shown on the lower spectra. To the contrary, people with no active infection will display spectra devoid of any signal, as illustrated in the upper spectra.
“It is very unusual to discover significant new human biochemical pathways, especially ones that might have major medical significance,” Professor Nicholson said.
Professor Wist said of the 10 X-VIP compounds detected, nine were new to human biology knowledge.
“These compounds are only produced during active viral infection, and they have very short half-lives so if we find them in the blood or urine of a patient then that patient is likely to be infectious and needs to isolate,” he said.
“The X-VIP metabolites represent previously unknown biomarkers of infection and infectiousness and may be highly relevant to rapid diagnosis in acutely infected hospitalised patients.
“Fascinatingly, from a chemical viewpoint these compounds ‘look’ like antiviral drugs and are highly similar to some compounds that are already marketed by drug companies as anti-viral agents.
“This X-VIP opens up the possibility of further studies aimed at designing new anti-viral drugs.”
Of particular note, according to Professor Nicholson, is that human biology contains the basic elements of antiviral drug synthesis and that this has been helping to protect humans since before human medicine existed.
Professor Wist said the compounds were detected in both urine and plasma, and a simple non-invasive urine test could also be developed for rapid detection.
“In fact, we have already demonstrated the proof of principle that we could deploy a rapid urine test for diagnosing active viral infections that could be of considerable medical significance,” he said.
Study lead Dr Samuele Sala said genetic evidence suggested the processes were ancient.
“It appears that nature created a mechanism for combatting viruses billions of years before humans even existed,” he said.
“Genomic evidence from other research indicates that the VIPERIN gene was present in very early organisms possibly including stromatolite ancestors, descendants of which coincidentally live off the coast of Western Australia.”
Professor Nicholson said COVID-19 had been subjected to unprecedented clinical and scientific scrutiny since emerging globally since late 2019.
COVID-19 presents with respiratory disease, but also impacts all major organs. For some people, these impacts – combined with prolonged immune system disruptions - can result in long-Covid or post-acute COVID-19 syndrome (PACS).
The ANPC has advanced global research in understanding the pathways to infection and the biological consequences of COVID-19, including long-Covid. The Centre deploys broad and deep metabolic analysis of blood plasma and urine samples using state of the art NMR (nuclear magnetic resonance), mass spectrometry and data modelling.
“The ANPC is examining the complex genetic, environmental and lifestyle interactions of the disease,” Professor Nicholson said.
“Understanding why the severity of symptoms in COVID-19 patients is so variable and discovering early markers that accurately define the likely impact on an individual patient, is critical to mitigating the health impacts on current patients, and responding effectively to other pandemic viruses which may emerge in future.”
This research supports the United Nations Sustainable Development Goal 3, Ensure healthy lives and promote well-being for all at all ages.
Discover more about how researchers at Murdoch University's Australian National Phenome Centre are working to revolutionise the diagnosis, prevention and treatment of serious health challenges.
News
COVID-19 researchers discover hidden natural immune defence pathway
Posted on
Health Futures Institute
The many interlinked facets of human health, from understanding the genome and its variable expression, to disease surveillance, health data linkage, mental health and navigating life's milestones, intersect at the Health Futures Institute.Learn more about our research.